The History of the Greenhouse Effect
An early science history from the 1800s through 1963
Revised and adapted from ‘The Discovery of Global Warming’, Spencer Weart (2008)
Origins of the Theory of the Greenhouse Effect
Early in the 1800s, the French scientist Joseph Fourier had asked himself a question. It was a deceptively simple question, of a sort that physics theory was just then beginning to learn how to address: what determines the average temperature of a planet like the Earth? When light from the Sun strikes the Earth’s surface and warms it up, why doesn’t the planet keep heating up until it is as hot as the Sun itself? Fourier’s answer was that the heated surface emits invisible ‘dark radiation’ (what we now call ‘infrared radiation’), which carries the heat energy away into space. If that was so, then why doesn’t Earth cool itself down all the way to the cold temperature of outer space?
The reason, Fourier intuited, was due to the Earth’s atmosphere, which somehow keeps some of the heat radiation in. He tried to explain this by comparing the Earth with its covering of air to a box covered with a pane of glass. The box’s interior warms up when sunlight enters and the heat cannot escape. The explanation sounded plausible, and several decades after Fourier's time, a few scientists had begun to speak of a ‘greenhouse effect’ that keeps the Earth from freezing. Though in fact it is a misnomer, for real greenhouses stay warm for other reasons (the main effect of the glass is to keep the air, heated by sun-warmed surfaces, from wafting away). As Fourier recognized, the way the atmosphere holds in heat on the entire Earth is more subtle. The atmosphere’s trick is to intercept a part of the infrared radiation emitted from the surface, preventing it from escaping into space. Jean-Baptiste Joseph Fourier, ‘On the Temperatures of the Terrestrial Sphere and Interplanetary Space’ (1824).
The correct reasoning for how Earth is kept warm was first explained lucidly by a British scientist, John Tyndall. Tyndall pondered how the atmosphere might control the earth’s temperature, but he was stymied by the opinion, held by most scientists at the time, that all gases are transparent to infrared radiation. In 1859 he decided to check this in his laboratory. He confirmed that the main gases in the atmosphere, oxygen and nitrogen, are indeed transparent. He was ready to quit when he thought to try coal gas. This gas, produced by heating coal and used for lighting, was piped into his laboratory. He found that for heat rays, the gas was as opaque as a plank of wood. Tyndall went on to try other gases, and he found that the gas CO2 was likewise opaque–what we would now call a ‘greenhouse’ gas.
A bit of CO2 is found in the Earth’s atmosphere, and although it is only a few parts in ten thousand, Tyndall saw how it could bring warming. Just as a sheet of paper will block more light than an entire pool of clear water, so the trace of CO2 altered the balance of heat radiation through the entire atmosphere. Much of the infrared radiation rising from the surface is absorbed by CO2 in the air. The heat energy is transferred into the air itself rather than escaping into space. Not only is the air warmed, but also some of the energy trapped in the atmosphere is radiated back to the surface and warms it. Thus, the temperature of the Earth is maintained at a higher level than it would be without the CO2. Tyndall put it neatly: ‘As a dam built across a river causes a local deepening of the stream, so our atmosphere, thrown as a barrier across the terrestrial [infrared] rays, produces a local heightening of the temperature at the Earth’s surface.’ John Tyndall, ‘Further Researches on the Absorption and Radiation of Heat by Gaseous Matter’ (1862), in Tyndall, Contributions to Molecular Physics in the Domain of Radiant Heat (New York: Appleton, 1873), p. 117.
Tyndall’s interest in all this had begun in a wholly different type of science. He hoped to solve a puzzle that was exciting great controversy among the scientists of his day: the prehistoric ice age. The claims were incredible, yet the evidence was clear. The scraped-down rock beds, the bizarre deposits of gravel found all around northern Europe and the northern United States—these looked exactly like the effects of Alpine glaciers, only immensely larger. Amid fierce debate, scientists were coming to accept an astounding discovery. Long ago—although not so long as geological time went, for Stone Age humans had lived through it—northern regions had been buried kilometers deep in continental sheets of ice. What could have caused this?
Changes in the atmosphere were one possibility, although not a promising one. Of the atmospheric gases, CO2 was not an obvious suspect, since there is so little of it in the atmosphere. The most important ‘greenhouse’ gas is \(\text{H}_2\text{O}\), simple water vapor. In his original experiments, Tyndall found that it readily blocks infrared radiation. He explained that water vapor ‘is a blanket more necessary to the vegetable life of England than clothing is to man. Remove for a single summer-night the aqueous vapor from the air . . . and the sun would rise upon an island held fast in the iron grip of frost.’ John Tyndall, ‘On Radiation through the Earth’s Atmosphere,’ Philosophical Magazine ser. 4, 25 (1863): 204–205. So if something dried out the atmosphere, that might cause an ice age. At present, Tyndall supposed, the atmosphere’s average humidity is maintained in some sort of automatic balance, in tandem with the global temperature.
The riddle of the ice age was taken up in 1896 by a scientist in Stockholm, Svante Arrhenius. Suppose, he said, the amount of CO2 in the atmosphere were changed. For example, a spate of volcanic eruptions might spew out vast quantities of the gas. This would raise the temperature a bit, and that small increment would have an important consequence: the warmer air would hold more moisture. Because water vapor is the truly potent greenhouse gas, the additional humidity would greatly enhance the warming. Conversely, if all volcanic emissions happened to shut down, eventually the CO2 would be absorbed into soil and ocean water. The cooling air would hold less water vapor. Perhaps the process would spiral into an ice age.
‘Cooling that causes less water vapor in the air that causes more cooling’ this is the kind of self-reinforcing cycle that today we call a ‘positive feedback’. The concept was both elementary and subtle—easy to grasp, but only after somebody pointed it out. In Arrhenius’s day only a few insightful scientists noticed that such effects could be crucial for understanding climate. The first important example had been worked out in the 1870s by a British geologist, James Croll, as he pondered possible causes of the ice age. When snow and ice had covered a region, he noted, they would reflect most of the sunlight back into space. Bare, dark soil and trees would be warmed by the Sun, but a snowy region would tend to remain cool. Once something started an ice age, the pattern could become self-sustaining.
Such complex effects were far beyond anyone’s ability to calculate at that time. The most Arrhenius could do was to estimate the immediate effects of changing the level of CO2. But he realized that he would also have to figure into his calculations the crucial changes in water vapor as the temperature rose or fell. The numerical computations cost Arrhenius month after month of tedious pencil work. He calculated the atmospheric moisture and the radiation entering and leaving the Earth for each zone of latitude. It seems he undertook the massive task partly as an escape from melancholy: he had just been through a divorce, losing not only his wife but custody of their little boy. The countless computations could hardly have been justified scientifically. Arrhenius had to overlook many features of the real world, and the data he used for how gases absorbed radiation were far from reliable. Nevertheless, he came up with numbers that he published with some confidence. If he was far from proving how the climate would change if CO2 varied, he did in truth get a rough idea of how it could change. He announced that cutting the amount of CO2 in the air by half would cool the world by about 6°C (and conversely that a doubling of CO2 would warm Earth by this much). Svante Arrhenius, ‘On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground’ (1896). That may not seem like a lot. But thanks to feedbacks, as extra snow accumulated and reflected sunlight, it could be enough to bring on an ice age.
Were such large changes in atmospheric composition possible? For that question Arrhenius turned to a colleague, Arvid Högbom. Högbom had compiled estimates for how CO2 cycles through natural geochemical processes—emission from volcanoes and uptake by the oceans and so forth—and he had come up with a strange new idea. It had occurred to him to calculate the amounts of CO2 emitted by factories and other industrial sources. Surprisingly, he found that the rate at which human activities were adding the gas to the atmosphere was roughly the same as the rates at which natural processes emitted and absorbed the gas. The added gas was not much compared with the volume of CO2 already in the atmosphere—the amount released from the burning of coal in the year 1896 would raise the level by scarcely a thousandth part. But the additions might matter if they continued long enough.
The idea of humans massively perturbing the atmosphere did not trouble Arrhenius. It was not just that warming seemed a good thing in chilly Sweden. Arrhenius, like nearly everyone at the end of the nineteenth century, expected any technological change would be for the best. Many people believed that scientists and engineers would solve all major problems in the centuries to come. In any case, Arrhenius figured it would take a couple of thousand years to double the amount of CO2 in the air. In his day barely a billion people populated the world, mostly peasants living like serfs. It scarcely seemed reasonable to imagine that humans could change the entire planet’s atmosphere, unless perhaps in some remote and fantastic future. Arrhenius had not quite discovered global warming, but only proposed a curious theoretical concept.
Scientific Skepticism of the Greenhouse Effect of CO2
Even as abstract theory, there were scientific reasons to dismiss Arrhenius’s idea. Most telling was a simple laboratory measurement made by another Swedish scientist, Knut Ångström (son of the more famous physicist Anders Ångström), that seemed to refute the entire principle of greenhouse warming. Ångström sent infrared radiation through a tube filled with CO2. The amount of radiation that got through the tube scarcely changed when he sharply cut the quantity of gas. He knew that CO2 absorbs radiation only in specific bands of the spectrum, and he reasoned that it took a mere trace of the gas to ‘saturate’ these absorption bands. The atmosphere was already so thoroughly opaque there that adding more gas could make little difference. Moreover, water vapor also absorbed infrared radiation in the same general region of the spectrum. The planet evidently already showed the maximum possible greenhouse effect. By 1910 most scientists thought Arrhenius’s original calculations were altogether wrong. (Though as we will see later, Ångström had made a big conceptual mistake thinking that the full multi-layered atmosphere operated like his single-compartment of gas experiment.)
To kill any lingering doubts, other scientists pointed out a still more fundamental objection. They held that it was impossible for CO2 to build up in the atmosphere at all. The relatively thin atmosphere contains only a little of the CO2 on the Earth’s surface, compared to the huge quantities locked up in rocks and in the oceans. For every molecule of CO2 in the air, there are about fifty dissolved in seawater. If humanity added more of the gas to the air, nearly all of it would eventually wind up in the oceans.
Furthermore, scientists saw that Arrhenius had grossly oversimplified the climate system in his calculations. For example, with more water vapor held in the air as the Earth got warmer, surely the moisture would make more clouds. The clouds would reflect sunlight back into space before the energy ever reached the surface, and so the Earth should hardly warm up after all.
These objections conformed to a view of the natural world that was so widespread that most people thought of it as plain common sense. In this view, the way cloudiness rose or fell to stabilize temperature and the way the oceans maintained a fixed level of gases in the atmosphere were examples of a universal principle: the Balance of Nature. Hardly anyone imagined that human actions, so small among the vast natural powers, could upset the balance that governed the planet as a whole. This view of Nature—suprahuman, benevolent, and inherently stable—lay deep in most human cultures. It was traditionally tied up with a belief in the fundamental stability and order of the universe, a flawless and imperturbable harmony. Such was the public belief, and scientists are members of the public, sharing most of the assumptions of their culture. Once scientists found plausible arguments explaining how the atmosphere and climate would remain unchanged within a human timescale—just as everyone expected—they stopped looking for possible counter-arguments.
Of course, everyone knew climate could vary. From the old folks’ tales of the great blizzards of their childhood to the devastating Dust Bowl drought of the 1930s, ideas about climate included a dose of catastrophe. But a catastrophe was (by definition) something transient; things revert to normal after a few years. A few scientists speculated about greater climate shifts. For example, had a waning of rainfall over centuries caused the downfall of ancient Near Eastern civilizations? Most doubted it. And if such changes really did happen, everyone assumed they randomly struck one region or another, not the entire planet.
To be sure, many knew there had been vast global climate changes in the distant past. Geologists were mapping out the ice age—or rather, ice ages. For it turned out that the tremendous sheets of ice had ground halfway down America and Europe and back not once, but over and over again. Looking still further in the past, geologists found a tropical age when dinosaurs basked in regions that were now Arctic. A popular theory suggested that the dinosaurs had perished when the Earth cooled over millions of years. The most recent ice age likewise had come to a gradual end, geologists reported, as the Earth returned to its present temperature over tens of thousands of years. If a new ice age was coming, it should take as long to arrive.
Ideals of consistency pervaded not only the study of climate, but also the careers of those who studied it. Through the first half of the twentieth century, climate science was a sleepy backwater. People who called themselves ‘climatologists’ were mostly bookkeepers who kept track of average seasonal temperatures, rainfall, and the like. Typical were the workers at the U.S. Weather Bureau, ‘the stuffiest outfit you’ve ever seen’, as one of a later generation of research-oriented geophysicists put it. Athelstan Spilhaus, interview by Ron Doel, Nov. 1989, American Institute of Physics, College Park, Md. Their job was to compile statistics on past weather, in order to advise farmers what crops to grow or tell engineers how great a flood was likely over the lifetime of a bridge. These climatologists’ products were highly appreciated by their customers (such studies continue to this day). And their tedious, painstaking style of scientific work would turn out to be indispensable for studies of climate change. Yet the value of this kind of climatology to society was based on the conviction that statistics of the previous half century or so could reliably describe conditions for many decades ahead. Textbooks started out by describing the term climate as a set of weather data averaged over temporary ups and downs. Climate was stable by definition.
Greenhouse Theory Restored
Nobody advised Gilbert Plass to study greenhouse warming. In the 1950s the Office of Naval Research supported his work of theoretical calculations for an experimental group at Johns Hopkins University that was studying infrared radiation. As Plass later recalled, he got curious about climate change only because he read broadly about topics in pure science. He happened on the discredited theory that the ice ages could be explained by changes in CO2. As a side project to his official work, Plass took to studying how CO2 in the atmosphere absorbed infrared radiation. Before he finished his analysis, he moved to southern California to join a group at the Lockheed Aircraft Corporation that was studying questions of infrared absorption directly related to heat-seeking missiles and other weaponry. Meanwhile, he wrote up his results on the greenhouse effect ‘in the evening,’ as he recalled, taking a break from his weapons research.
Plass knew the old objection to the greenhouse theory of climate change: in the parts of the spectrum where infrared absorption took place, the CO2 and water vapor that were already in the atmosphere sufficed to block all the radiation that could ever be blocked, so a change in the level of the gas could not matter. However, doubts about this had been raised during the 1940s by new measurements and an improved theoretical approach. In fact, Ångström’s old measurements had been faulty: the CO2 absorption bands are not totally saturated, and they do not entirely overlap the water vapor bands. More important, a change in gas levels would matter even if all heat radiation was already blocked in the lower atmosphere. After all, the surface of the planet would heat up without limit unless it somehow shed the energy received from the Sun. The heat energy must work its way up through the atmosphere, layer by layer, until it reaches the thin upper layers where radiation does escape easily into space. In the frigid and rarefied air up there, the broad bands that tended to block radiation at sea level resolved into clusters of narrow spectral lines, like a picket fence with spaces where radiation could slip through. Plass realized that adding more CO2 in an upper layer would indeed make a difference. Like layering more and more blankets on yourself, you will continue to get warmer.
Plass could say nothing more specific without extensive computations. Fortunately, he had access to newly invented digital computers. His lengthy calculations demonstrated that adding or subtracting some CO2 could make a difference, seriously reducing or increasing the amount of radiation that escaped from the Earth’s surface into space. In 1956 Plass announced that current human activity would raise the average global temperature ‘at the rate of 1.1 degree C per century.’ G. N. Plass, ‘Carbon Dioxide and the Climate,’ American Scientist 44 (1956): 302–316.
Plass’s computation was too crude to convince other scientists, for he had left out crucial factors such as possible changes in water vapor and clouds. But he did prove a central point: the greenhouse effect could not be dismissed with the old argument that adding more CO2 could make no difference. He warned that climate change could be ‘a serious problem to future generations’, although not for several centuries. Like Tyndall and Arrhenius, Plass was chiefly interested in the mystery of the ice ages. If the world’s temperature continued to rise to the end of the century, he thought it would matter mainly to scientists, by confirming the CO2 theory of climate change. G. N. Plass, ‘Carbon Dioxide and the Climate,’ American Scientist 44 (1956): 302–316.
Geochemistry, the Ocean, and the Carbon Cycle
Another question was whether the level of CO2 was gradually rising, as Plass suspected. That question had little chance to attract research funds. Experts believed any rise of CO2 would be too slow to matter for a long time to come, and it probably couldn’t happen at all. For if Plass had shown that the facts of infrared absorption did not rule out greenhouse warming, another weighty objection to the theory remained. Wouldn’t the oceans simply swallow up whatever extra CO2 we humans might pour into the atmosphere?
It happened that the movements of carbon could now be tracked with a new tool, radiocarbon—a radioactive isotope, carbon-14. Such isotopes were studied during the wartime work to build nuclear weapons, and the pace had not slackened in the postwar years. Sensitive instruments were developed to detect radioactive fallout from Soviet nuclear tests, and a few scientists turned the devices to measuring radiocarbon. Their studies also drew support from interests far removed from the Cold War. Archeologists were fascinated by the way radiocarbon measurements could give exact dates for ancient relics such as mummies or cave bones. The carbon-14 isotope is created in the upper atmosphere, when cosmic-ray particles from outer space strike nitrogen atoms and transform them into radioactive carbon. Some of the radiocarbon finds its way into living creatures. After a creature’s death, carbon-14 slowly decays at a fixed rate over hundreds and thousands of years to the stable nitrogen-14. Thus, the less of it that remains in an object, in proportion to normal carbon (carbon-12), the older the object is.
One of the new radiocarbon experts, the chemist Hans Suess, thought of applying the technique to the study of geochemistry. It occurred to him that the carbon emitted when humans burned fossil coal and oil is ancient indeed, its radioactive carbon-14 nearly all gone. He gathered wood from century-old trees and compared it with modern samples. In 1955 Suess announced that he had detected that ancient carbon (with depleted carbon-14 levels) had been added to the modern atmosphere, presumably from the burning of fossil fuels. But he thought that the fossil fuel carbon made up barely 1 percent of all the carbon in the atmosphere—a figure so low that he concluded that most of the carbon derived from fossil fuels was being promptly taken up by the oceans. A decade would pass before he managed to get more accurate measurements, which would show a far higher fraction of fossil carbon.
Everyone knew that radiocarbon measurements were tricky; Suess’s data were obviously preliminary and uncertain. The important thing he had demonstrated was that fossil carbon had shown up in the atmosphere. With more work one might figure out exactly how long it would take the oceans to absorb the carbon derived from burning fossil fuels. The question was intriguing, for as an oceanographer admitted, ‘Nobody knows whether it takes a hundred years or ten thousand.’ Roger Revelle, ‘The Oceans and the Earth,’ talk given at American Association for the Advancement of Sciences symposium, 27 Dec. 1955.
This oceanographer was Roger Revelle, a dynamo of a researcher and administrator who was driving the expansion of the Scripps Institution of Oceanography near San Diego, California. Sitting on a dramatic cliff overlooking the Pacific, the prewar Scripps had been a typical oceanographic establishment, quiet and isolated, with its small clique of a dozen or so gossiping researchers and a single research ship. It relied on private patronage, which faltered when the Depression bit into the Scripps family’s funds. The postwar Scripps was growing into something quite different, a complex of modern laboratories. Revelle won funding for a variety of projects under contracts from the Office of Naval Research, the natural patron for any research related to the oceans. One of Revelle’s many good ideas was to use some of the money to hire Suess to come to Scripps and pursue radiocarbon studies. By December 1955 the two had joined forces, combining their expertise to study carbon in the oceans.
From measurements of radiocarbon in seawater and air, Suess and Revelle deduced that the ocean surface waters took up a typical molecule of CO2 from the atmosphere within a decade or so. Other scientists who looked into the question around the same time confirmed the conclusion. Yes, the oceans were absorbing most of the carbon humanity added to the atmosphere. Revelle and Suess wrote this up for publication.
The only question left, it seemed, was whether it would accumulate near the surface, or whether currents would carry it deep into the oceans. Revelle’s group was already studying the question of how fast the ocean surface waters turned over. It was a matter of national interest, for the navy and the U.S. Atomic Energy Commission were concerned about the fate of nuclear fallout from bomb tests. The Japanese were in an uproar over contamination of the fish they relied on. Moreover, if the ocean currents were slow enough, radioactive waste from nuclear power plants could be dumped on the ocean floor. Measurements of radiocarbon at various depths and other studies, pursued at Scripps and elsewhere in the 1950s, showed that on average the ocean waters turn over completely in several hundred years. That seemed fast enough to sweep the CO2 produced by human industry into the depths of the ocean.
But the ocean is not just salt water, but a complex mix of chemicals, so it was not obvious that seawater could actually hold on to the CO2 it absorbed. The mix of chemicals in seawater creates what chemists call a buffering mechanism, which stabilizes the water’s acidity. This had been known for decades, but nobody had realized what it meant for CO2. Now Revelle saw that when some molecules were absorbed, their presence would alter the chemical balance through a chain of reactions. Although it was true that most of the CO2 molecules added to the atmosphere would wind up in ocean surface water within a few years, most of these molecules (or others already in the oceans) would promptly be evaporated back out. Revelle calculated that in sum, the ocean surface could not really absorb much gas—barely one-tenth the amount his earlier calculations had predicted.
It was now 1957, and Revelle and Suess were ready to send off for publication their paper describing how the oceans would promptly absorb all the extra CO2 humanity could produce. Revelle went back and added a few sentences to explain why this would not in fact occur. As sometimes happens with landmark scientific papers, written in haste while understanding just begins to dawn, Revelle’s self-contradictory discussion was so obscure that it took other scientists a couple of years to understand and accept his discovery. Revelle himself did not grasp all the implications at first. In the revised paper he included a quick calculation that the amount of CO2 in the atmosphere would rise gradually over the next few centuries, then level off with a total increase of 40 percent or less.
That reassuring conclusion was a gross underestimate. Revelle was assuming that industrial emissions during the coming centuries would continue at the 1957 rate. Scarcely anyone had yet understood that both population and industrialization were exploding in exponential growth. Between the start of the twentieth century and its end, the world’s population would quadruple, and the use of energy by an average person would quadruple, making a sixteen-fold increase in the rate of emission of CO2. Yet at midcentury, world wars and the Great Depression had led most technologically advanced nations to worry about a possible decline in their populations. Their industries seemed to be plodding ahead, expanding no faster in that decade than in the last. As for ‘backward’ regions like China and Brazil, industrialization had scarcely entered anyone’s calculations except as a possibility for the remote future.
Different ideas were beginning to stir. The geochemist Harrison Brown was sketching out a vision of a future with exploding population and industrialization. Revelle had heard these ideas, and before he sent off for publication the paper he had written with Suess, he added a remark: the accumulation of CO2 ‘may become significant during future decades if industrial fuel combustion continues to rise exponentially.’ In conclusion, he wrote, ‘Human beings are now carrying out a large scale geophysical experiment of a kind that could not have happened in the past nor be reproduced in the future.’ Roger Revelle and Hans E. Suess, ‘Carbon Dioxide Exchange between Atmosphere and Ocean and the Question of an Increase of Atmospheric CO2 during the Past Decades,’ Tellus 9 (1957): 18–27.
Revelle meant ‘experiment’ in the traditional scientific sense, a nice opportunity for the study of a geophysical process. Yet he did recognize that there might be some future risk. Other scientists too began to feel a mild concern as they gradually assimilated the meaning of Plass’s and Revelle’s difficult calculations. Adding CO2 to the atmosphere could change the climate after all. And the changes might arrive not in some remote science-fiction future, but within the next century or so.
Revelle and Suess, like Arrhenius, Plass, and everyone else who up till then had made a contribution toward the discovery of global warming, had taken up the question as a side issue. They saw in it a chance for a few publications, a detour from their main professional work, to which they soon returned. If just one of these men had been possessed by a little less curiosity, or a little less dedication to laborious thinking and calculation, decades more might have passed before the possibility of global warming was noticed. It was also a historical accident that military agencies were scattering money with a fairly free hand in the 1950s. Without the Cold War there would have been little funding for research on a subject nobody had connected with practical affairs. The U.S. Navy had bought an answer to a question it had never thought to ask.
Revelle and Suess were now eager to learn more about the ‘large scale geophysical experiment’ of greenhouse warming. Few others paid much attention to their difficult technical paper. Government agencies like the navy could hardly be expected to devote much more money to a question that seemed unlikely to yield anything useful, or even more scientific information, without great effort. Fortunately, just then another purse opened up. The new funds came (or seemed to come) from peaceful internationalism, altogether apart from national military drives.
Measuring Atmospheric CO2
After the Second World War, governments saw new reasons to support international cooperation in science. This was the era that saw the creation of the United Nations, the Bretton Woods financial institutions, and many other multilateral efforts. The aim was to bind peoples together with interests that transcended the self-serving nationalism. In the mid-1950s a small band of scientists worked out a scheme to boost cooperation among the various geophysics disciplines. They hoped to coordinate data gathering on an international scale and—no less important—to persuade governments to add an extra billion or so dollars to their funding of geophysics research, thereby allowing for the hiring of more scientist researchers and funding for more experiments. Their plans culminated in the creation of the International Geophysical Year (IGY) of 1957–1958. The IGY would draw together scientists from many nations and a dozen different disciplines, to interact in committees that would plan and carry out interdisciplinary research projects grander than any attempted before.
Climate change ranked low on the list of IGY priorities. But with such a big sum of new money, there was bound to be something for climate-related topics. In the committees that allocated the U.S. share of funding, Revelle and Suess argued for a modest program to measure the gas in the ocean and air simultaneously at various points around the globe. It wouldn’t cost much, so the committee granted some money.
Revelle had David Keeling in mind for this work. Keeling was a young geochemist interested in understanding the processes that affect the level of CO2 in the atmosphere and what that level of CO2 could in turn affect. Revelle hired Keeling to come to Scripps and conduct the world survey. Revelle wanted to establish a baseline ‘snapshot’ of CO2 levels around the world, averaging over the large variations observed from place to place and from time to time. After a couple of decades, somebody could come back, take another snapshot, and see if the average CO2 level had risen.
Keeling thought he could do better than that. The greatest accuracy called for expensive new instruments, far more precise than most experts thought were necessary to measure something that fluctuated as widely as CO2 levels. Keeling lobbied key officials and managed to persuade them to give him money for the instruments. He set one up atop the volcanic peak Mauna Loa in Hawaii, surrounded by thousands of miles of clean ocean, one of the best sites on Earth to measure the undisturbed atmosphere. Another instrument went to the even more pristine Antarctic.
Keeling’s costly equipment, together with his relentless pursuit of every possible source of error, paid off. In Antarctica, he tracked down variations in the CO2 measurements to emissions from nearby machinery. On Mauna Loa, gas leaking from vents in the volcano itself was to blame. Stalking such problems with meticulous attention to detail, Keeling found a remarkably precise and consistent baseline number for the level of CO2 in the atmosphere. His first twelve months of data hinted that a rise could be seen in just that one year.
But the IGY was winding down. By November 1958 the remaining funds had fallen so low that CO2 monitoring would have to stop. Keeling scrambled to find more money. Suess and Revelle diverted a fraction from a grant that the Atomic Energy Commission had given Scripps for other purposes. (In those days, more than now, agencies trusted scientists to spend funds as they chose.) In 1960, with two full years of Antarctic data in hand, Keeling reported that the baseline CO2 level had risen. Charles D. Keeling, ‘The Concentration and Isotopic Abundances of Carbon Dioxide in the Atmosphere,’ Tellus 12 (1960): 200–203. The rate of the rise was approximately what would be expected if the oceans were not swallowing up most industrial emissions.
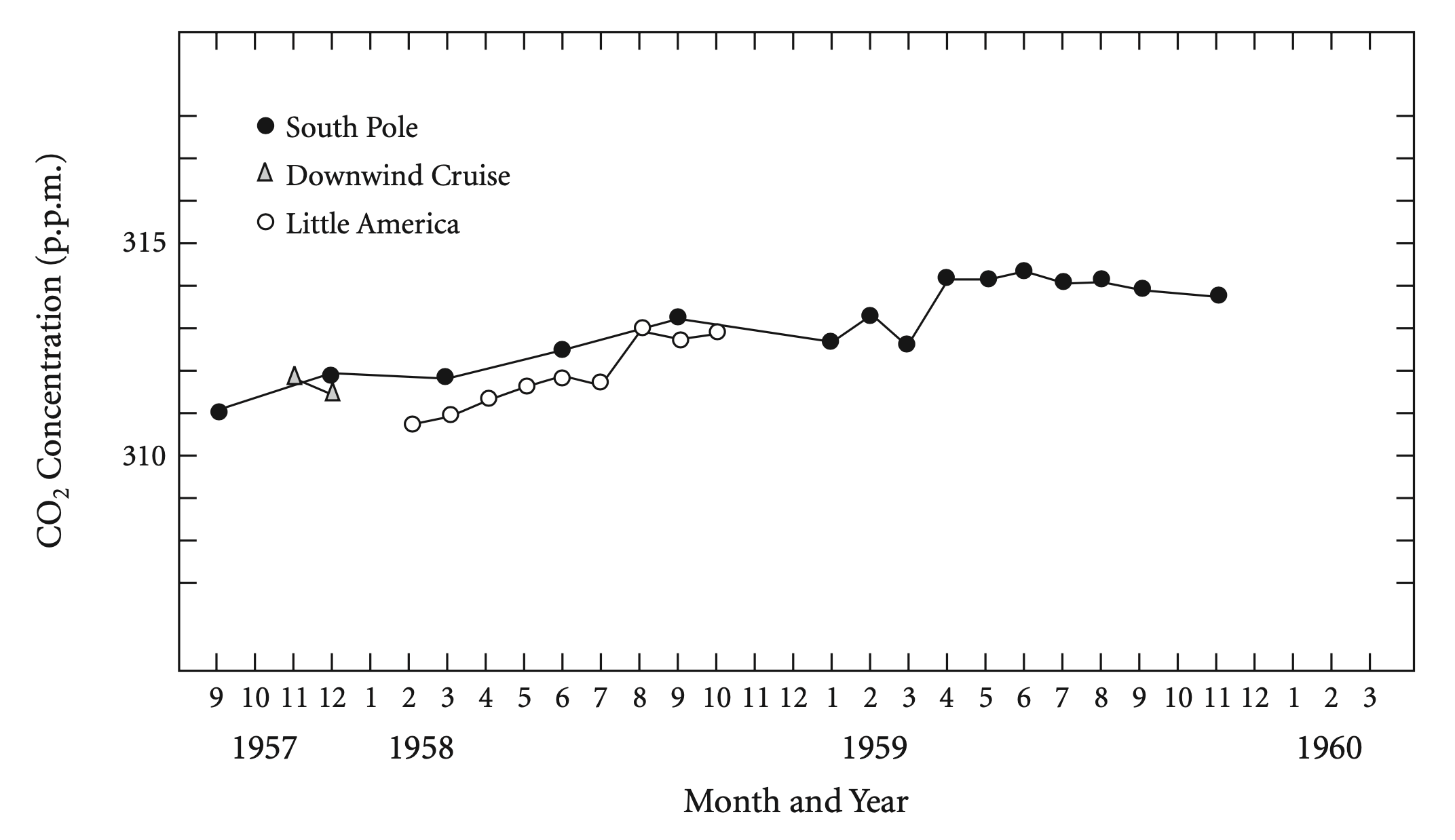
Although some scientists immediately recognized the importance of Keeling’s work, no agency felt responsible for funding a climate study that might run for many years. In 1963 the work almost had to shut down. Keeling turned to the National Science Foundation, a U.S. federal agency established back in 1950 on a modest budget. The 1957 launching of the Soviet Sputnik satellite had spurred Americans, fearful of trailing behind their Communist enemy, to boost funding for all areas of science. The National Science Foundation took over from military agencies much of the nation’s support of basic research. One minor consequence was that Keeling got funds to continue the Mauna Loa measurements.
As the Mauna Loa data accumulated, the record grew increasingly impressive, showing CO2 levels noticeably higher year after year. What had begun as a temporary job for Keeling was turning into a lifetime career—the first of many careers that scientists would eventually dedicate to climate change. Within a few years, Keeling’s inexorably rising CO2 curve was widely cited by scientific review panels and science journalists. It became the central icon of the greenhouse effect.
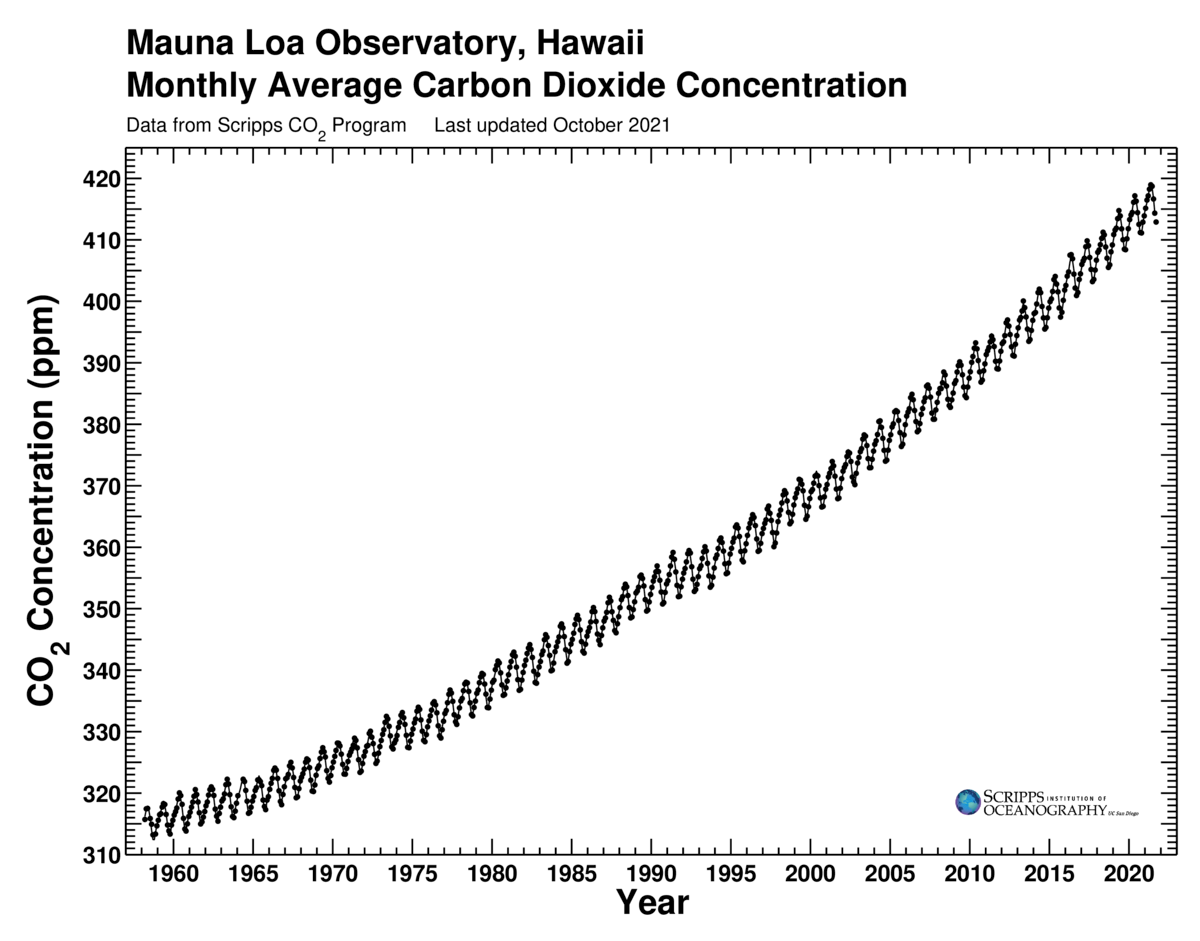
Keeling’s data put a capstone on the structure built by Tyndall, Arrhenius, Plass, and Revelle and Suess. This was not quite the discovery of global warming. It was the discovery of the possibility of global warming. Experts would continue for many years to argue over what would actually happen to the planet’s climate. But no longer could a well-informed scientist dismiss out of hand the possibility that our emission of greenhouse gases would warm the Earth. That odd and unlikely theory now emerged from its cocoon, taking flight as a serious research topic.
\(\Uparrow\) To the top
\(\Rightarrow\) Next chapter
\(\Leftarrow\) Table of contents